

To get a closer, real-time look at developing fetuses, and to better understand the potential causes of birth defects and other health issues, scientists have turned to a source you might not have expected: quail eggs.
In fact, our earliest developments as living beings are similar to those of quails, and because their embryos grow inside eggs, they can be scanned relatively easily. Avian eggs have long been favored by scientists for the study of embryos.
Here, researchers in Australia used eggs carrying quails bred to express a fluorescent peptide that binds to actin proteins that form the structure of the early embryo, called the actin cytoskeleton. This approach allowed them to watch cells migrating and coming together to form organs.
“For the first time we have seen high-resolution, real-time imaging of important early developmental processes,” says developmental biologist Melanie White from the University of Queensland.
“Until now, most of our knowledge of post-implantation development came from studies on static slides, at fixed points in time.”
frameborder=”0″ allow=”accelerometer; autoplay; clipboard-write; encrypted-media; gyroscope; picture-in-picture; web-share” referrerpolicy=”strict-origin-when-cross-origin” allowfullscreen>
The team was able to see the very early stages of heart, brain, and spinal cord formation. A variety of microscope instruments were used to capture the fluorescent marker, which outlined the movement of cells.
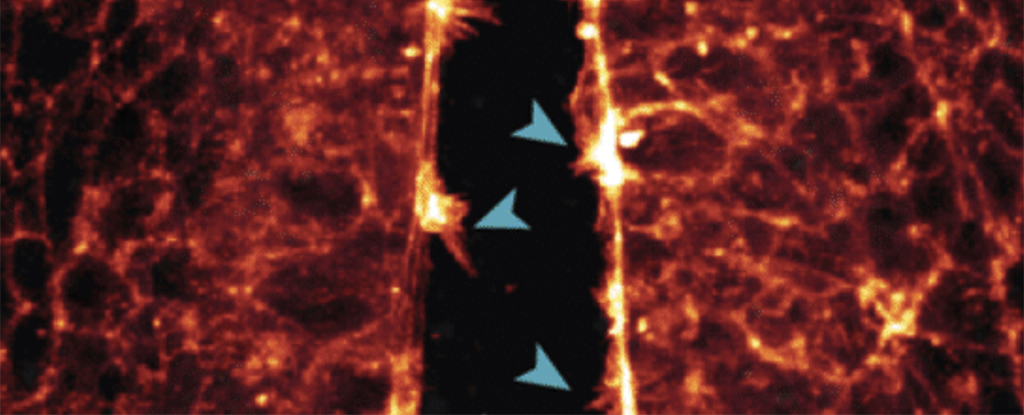
One of the observations made was of the neural tube, the precursor to the central nervous system, being ‘zipped up’ as cells joined together.
“We saw how the cells reached across the open neural tube with their protrusions to contact the opposite side – the more protrusions the cells formed, the faster the tube zipped up,” White explains.
“If this process goes awry or is disrupted and the tube doesn’t close properly during the fourth week of human development, the embryo will have brain and spinal cord defects.”
There were similar connections made in the stem cells that would eventually form the hearts of the quails.
“We were able to image the filopodia from heart stem cells deep inside the embryo as they first made contact by sticking out protrusions and gripping to their surroundings and each other to form the early heart,” says White.
“It’s the first time anyone has captured the cell’s actin cytoskeleton facilitating this contact in live imaging.”
Besides offering a fascinating insight into early life, the study is important for growing our knowledge of how and why birth defects occur. When the connection processes fail, that can lead to problems for the developing infant.
Seeing these biological transformations take place in real time, and at the smallest scales, should be useful in the future for mitigating or at least identifying the risk of birth defects. Plenty more studies of quail eggs using this process are now planned by the team.
Scientists are continuing to improve their models and their understanding of what happens in the womb, and through that we can work to make more pregnancies as healthy as possible.
“Our aim is to find proteins or genes that can be targeted in the future or used for screening for congenital birth defects,” says White.
“We are very excited at the possibilities that this new quail model now offers to study development in real time.”
The research has been published in The Journal of Cell Biology.